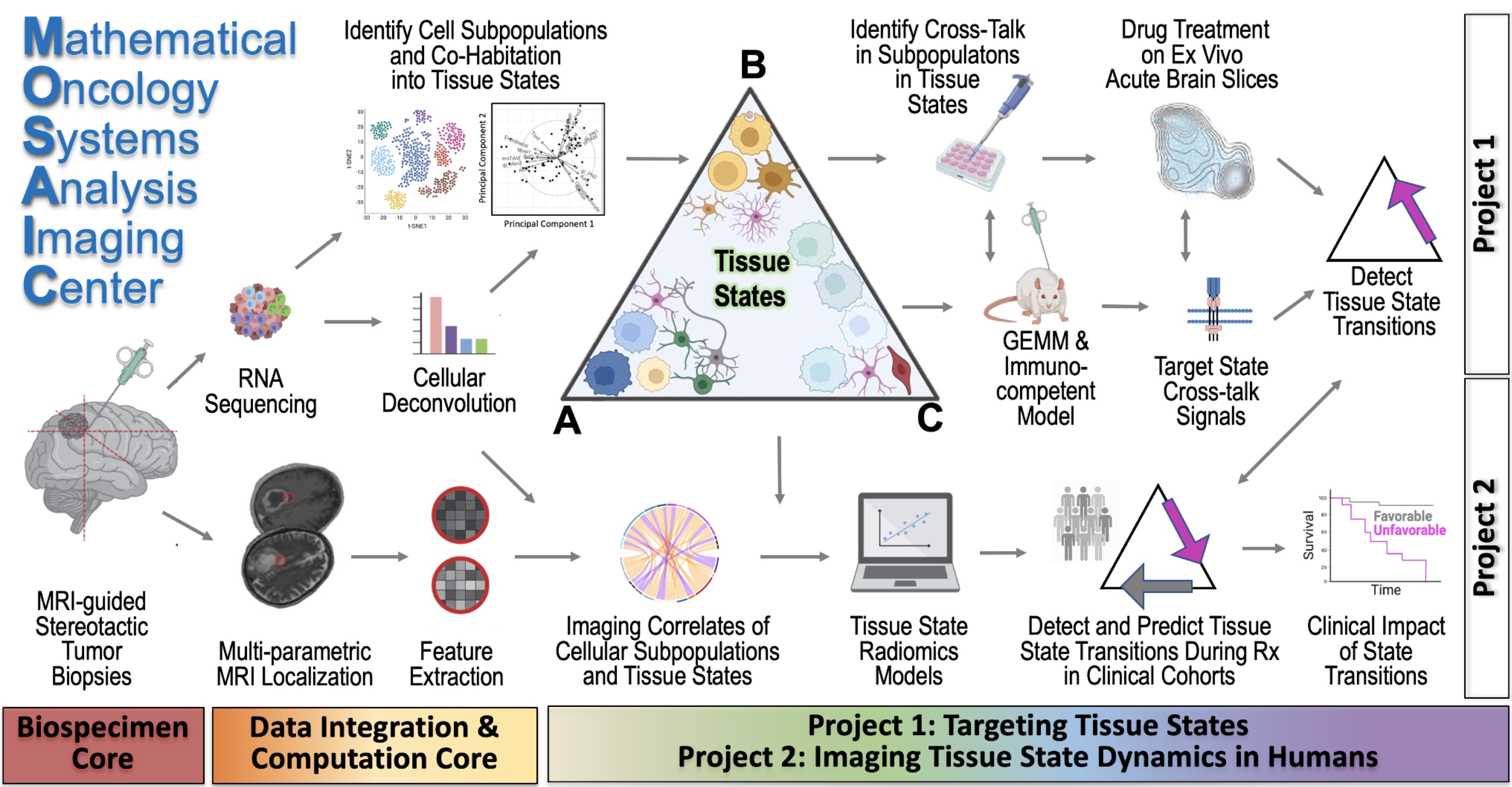
Latest Article Published in Nature Methods
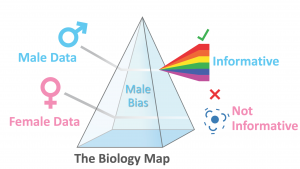 We recently published a paper highlighting a major oversight with regard to sex bias in the maps used pervasively in biology and medicine. These maps are used by essentially everyone in biology and medicine. The maps come in the form of big databases that tell us how the basic elements of biology (e.g. genes, proteins, etc) are *thought to be* connected together. Yet, our article clarifies that these maps have failed to incorporate sex into their curation so there is no easy way to know if the maps in these databases are hiding sex-based differences in biology!
We recently published a paper highlighting a major oversight with regard to sex bias in the maps used pervasively in biology and medicine. These maps are used by essentially everyone in biology and medicine. The maps come in the form of big databases that tell us how the basic elements of biology (e.g. genes, proteins, etc) are *thought to be* connected together. Yet, our article clarifies that these maps have failed to incorporate sex into their curation so there is no easy way to know if the maps in these databases are hiding sex-based differences in biology!
Sara Ranjbar, PhD awarded Edward C. Kendall Mayo Clinic Alumni Award for Meritorious Research
Congratulations Sara on being the 2021 recipient of the Edward C. Kendall Mayo Clinic Alumni Award for Meritorious Research!!!!!
Presented annually, the Mayo Clinic Alumni Association Edward C. Kendall Alumni Award for Meritorious Research is awarded in recognition of outstanding research conducted by an individual whose primary appointment is in research. Recipients receive an award of $2,000 and a certificate of accomplishment from the Mayo Clinic Alumni Association.
We are so very proud of you!!!
New NCI PSOC U01 Funded
Excited to finally announce our new funding of the NCI U01 grant CA25048 entitled: “Image-based models of tumor-immune dynamics in glioblastoma”
Contact MPI: Kristin Swanson
MPI: Leland Hu, MD (Radiology, Mayo Clinic)
MPI: Peter Canoll, MD, PhD (Director of Neuropathology, Columbia Univ)
This $3.8M award will support our ability to resolve the dynamics of the human immune system within the glioblastoma microenvironment during the course of a patients’ care.
Abstract: Our inability to explain why some patients do not respond to immunotherapy, combined with our inability to
predict the responders, poses a serious challenge to advancing cancer care and research. Currently, biopsies
serve as the most informative way to assess the immunological activity within a cancerous area, but we are
spatially and temporally limited in the number of biopsies we can obtain from patients, especially in cases of
brain cancer. We propose here a novel strategy merging artificial intelligence and mechanistic modeling to
reveal the spatial and temporal tumor-immune landscape within patients by connecting a unique resource of
image-localized biopsies and clinical imaging for cohorts treated with standard and immunotherapies.
Dr. Swanson on the Finding Genius Podcast
Dr. Swanson Named the Vasek and Anna Maria Polak Professor in Cancer Research
Lab Presents Posters at the Annual Society for Neuro-Oncology Meeting
The Annual Society for Neuro-Oncology Meeting was held virtually this year and our lab presented two posters:
- Sex differences in GBM treatment response: an observational study.
Authors: Julia Lorence, Tomas Bencomo, Haylye White, Cassandra R. Rickertsen, Susan C. Massey, Kyle W. Singleton, Andrea Daruud-Hawkins, Sandra K. Johnston, Alyx B. Porter, Maciej M. Mrugala, Bernard R. Bendok, Leland Hu, Joshua B. Rubin, and Kristin R. Swanson
- Leveraging Transcriptome Sequencing and Mathematical Modeling to Investigate Glioblastoma-Macrophage Interactions
Authors: Javier C. Urcuyo, Andrea Hawkins-Daarud, Susan Christine Massey, Jeffrey N. Bruce, Peter D. Canoll, Nhan L. Tran, Leland S. Hu, and Kristin R. Swanson
Mathematical Oncology Blogpost – Thriving and Evolving During the Pandemic, by Andrea Hawkins-Daarud
Thanks to Covid-19, many of us have found ourselves working from home. Indeed as a lab, we have had to adjust to this new setup. Andrea Hawkins-Daarud (one of our Associate Directors) has recently published a mathematical oncology blogpost, outlining the ways in which we’ve shifted our work style in response to the pandemic. Check out the link below!
Dr. Swanson Featured as a “Unicorn Doctor” in Phoenix Magazine’s “Top Docs” Issue.
As our world faces the dramatic challenge of #COVID19, we appreciate Phoenix Magazine for recognizing the importance of innovative approaches to medical challenges including our Mathematical Oncology work.
https:://www.phoenixmag.com/2020/03/19/unicorn-doctors/
Lab to Present 16 Abstracts at the Annual Society for NeuroOncology Meeting
Our lab members and collaborators are presenting 14 abstracts at the Annual Society for NeuroOncology Meeting (click on title for abstract):
- Cystic glioblastoma presentation as a beneficial prognostic indicator for overall survival. Saturday, Nov 23: 5:00 PM – 7:00 PM Ballroom Lawn
Authors: Lee Curtin, Paula Whitmire, Cassandra R Rickertsen, Peter Canoll, Maciej M Mrugala, Kristin R. Swanson and Leland S. Hu
- Lacunarity and fractal dimension act as pretreatment imaging biomarkers for survival in glioblastoma patients. Saturday, Nov 23: 5:00 PM – 7:00 PM Ballroom Lawn
Authors: Lee Curtin, Paula Whitmire, Haylye White, Maciej M. Mrugala, Leland S. Hu and Kristin R. Swanson
- Revealing the Tumor-Immune Landscape Through Spatially-Resolved Radiomics: Case Studies. Saturday, Nov 23: 5:00 PM – 7:00 PM Ballroom Lawn
Authors: Andrea Hawkins-Daarud, Hyunsoo Yoon, Dileep Monie, Kyle Singleton, Sara Ranjbar, Nhan Tran, Behnam Badie, Russell C. Rockne, Christine Brown, Leland Hu, Jeffrey Bruce, Peter Canoll, Jing Li and Kristin R. Swanson
- Does the presence of gadolinium affect T2 –weighted images of glioma?: Implications for MRI protocol standardization. Friday, Nov 22: 7:30 PM – 9:30 PM Ballroom Lawn
Authors: Pamela R. Jackson, Yuxiang Zhou, Joseph Hoxworth, Kristin R. Swanson and Leland S. Hu
- Sex Differences in Contrast-Enhancing Gliomas at Presentation. Friday, Nov 22: 7:30 PM – 9:30 PM Ballroom Lawn
Authors: Sandra K. Johnston, Aditya Khurana, Paula Whitmire, Akanksha Sharma, Andrea Hawkins-Daarud, Sara Ranjbar, Leland S. Hu, Joshua B. Rubin, Kathleen M. Egan, Peter D. Canoll, Alyx B. Porter, Maciej M. Mrugala, Priya Kumthekar and Kristin R. Swanson.
- Impact of tumor location on image-derived volume, proliferation rate and growth velocity in glioblastoma patients. Friday, Nov 22: 7:30 PM – 9:30 PM Ballroom Lawn
Authors: Julia Lorence, Sara Ranjbar, Paula Whitmire, Cassandra R. Rickertsen, Sandra K. Johnston, Haylye White, Kyle W. Singleton, Kamala Clark-Swanson, Bernard R. Bendok, J. Ross Mitchell, Alyx B. Porter, Maciej M. Mrugala, Joshua B. Rubin, Leland S. Hu and Kristin R. Swanson
- Sex differences in imaging-based assessment of glioblastoma invasion. Saturday, Nov 23: 5:00 PM – 7:00 PM Ballroom Lawn
Authors: Susan Christine Massey, Andrea Hawkins–Daarud, Spencer Bayless, Pamela R. Jackson, Rebecca Grove, Katrina Bakken, Lihong He, Ann C. Mladek, Ashley Stokes, Ashlyn C. Gonzales, Ashley Nespodzany, Jennifer Eschbacher, Leslie C. Baxter, Kris A. Smith, Peter Nakaji, Samuel McGee, Bernard R. Bendok, Terence Burns, Jann N. Sarkaria, Leland S. Hu, Kristin R. Swanson1
- Detection of cystic glioblastoma from magnetic resonance imaging using deep learning techniques. Saturday, Nov 23: 5:00 PM – 7:00 PM Ballroom Lawn
Authors: Sara Ranjbar, Lee Curtin, Paula Whitmire, Leland S Hu, Kristin R. Swanson
- Evaluating the Days Gained response metric in small clinical trials using bevacizumab plus additional agents for recurrent glioblastoma. Friday, Nov 22: 7:30 PM – 9:30 PM Ballroom Lawn
Authors: Kyle W. Singleton, Sandra K. Johnston, Cassandra R. Rickertsen, S. Keith Anderson, Gustavo De Leon, Scott A. Whitmire, Kamala R. Clark-Swanson, Bernard R. Bendok, Maciej M. Mrugala, Alyx B. Porter, Evanthia Galanis, Kristin R. Swanson
- Quantifying individualized ABT-414 sensitivity and blood-brain barrier penetrance from serial imaging of patient-derived xenografts models of glioblastoma. Friday, Nov 22: 7:30 PM – 9:30 PM Ballroom Lawn
Authors: Javier Urcuyo, Susan Christine Massey, Bianca Maria Marin, Jann Sarkaria and Kristin R. Swanson
- T2-weighted imaging may be indicative of drug distribution in glioblastoma patients. SNO-SCIDOT
Authors: Pamela R. Jackson, Minjee Kim, Andrea Hawkins-Daarud, Kyle Singleton, Afroz Mohammad, Terence Burns, Ian Parney, Leland S. Hu, Timothy Kaufmann, William Elmquist, Jann Sarkaria and Kristin R. Swanson
- Identifying the spatial and temporal dynamics of glioblastoma subpopulations within individual patients. Saturday, Nov 23: 5:52 PM – 5:56 PM Wildflower B
Authors: Bethan Morris, Lee Curtin, Andrea Hawkins-Daarud, Bernard Bendok, Maciej M. Mrugala, Jing Li, Nhan Tran, Leland S. Hu, Matthew Hubbard, Markus R. Owen, Kristin R. Swanson
- Predicting Seizure in Glioma Patients Using a Random Forest Classifier Trained on Sex-Specific and Mixed Cohorts. Saturday, Nov 23: 5:00 PM – 7:00 PM Ballroom Lawn
Authors: Aditya Khurana, Sandra K. Johnston, Paula Whitmire, Sara Ranjbar, Akanksha Sharma, Andrea Hawkins-Daarud, Joshua B Rubin, Alyx B. Porter, Peter D. Canoll, Kathleen M. Egan, Leland S. Hu, Maciej M. Mrugala, Priya Kumthekar, Kristin R. Swanson
- Reproducible radiomic mapping of tumor cell density by machine learning and domain adaptation. Friday, Nov 22: 7:30 PM – 9:30 PM Ballroom Lawn
Authors: Lujia Wang, Hyunsoo Yoon, Andrea Hawkins-Daarud, Kyle W. Singleton, Kamala R. Clark-Swanson, Bernard R. Bendok, Maciej M. Mrugala, Jenny Eschbacher, Kris A. Smith, Peter Nakaji, Ashlyn C. Gonzalez, Ashley Nespodzany, Leslie C. Baxter, Teresa Wu, Kristin R. Swanson, Leland S. Hu and Jing Li
- Uncertainty quantification in radiomics. Friday, Nov 22: 7:30 PM – 9:30 PM Ballroom Lawn
Authors: Lujia Wang, Hyunsoo Yoon, Andrea Hawkins-Daarud, Kyle W. Singleton, Kamala R. Clark-Swanson, Bernard R. Bendok, Maciej M. Mrugala, Jenny Eschbacher, Kris A. Smith, Peter Nakaji, Ashlyn C. Gonzalez, Ashley Nespodzany, Devi Patra, Gustavo De Leon, Richard S. Zimmerman, Alyx B. Porter, Chandan Krishna, Marcela Salomao, Joseph Hoxworth, Yuxiang Zhou, Maciej M. Mrugala, Nhan L. Tran, Teresa Wu, Kristin R. Swanson, Jing Li, Leland S. Hu,
- Using machine learning to build radiomics models that distinguish regions of glioblastoma recurrence vs tumor progression on MRI.
Authors: Hyunsoo Yoon, Andrea Hawkins-Daarud, Akshay Save, Kyle W. Singleton, Kamala R. Clark-Swanson, Lujia Wang, Bernard R. Bendok, Maciej M. Mrugala, Teresa Wu, Jeffrey Bruce, Leland S. Hu, Jing Li, Peter D. Canoll and Kristin R. Swanson
