New method is first to predict brain cancer outcome and quickly show if therapy is effective
CHICAGO — The critical question shortly after a brain cancer patient starts treatment: how well is it working? But there hasn’t been a good way to gauge that.
Now Northwestern Medicine researchers have developed a new method — similar to forecasting storms with computer models — to predict an individual patient’s brain tumor growth. This growth forecast will enable physicians to rapidly identify how well the tumor is responding to a particular therapy. The approach allows a quick pivot to a new therapy in a critical time window if the current one isn’t effective.
The study is based on 33 patients with glioblastoma, the most common and aggressive form of brain cancer. The paper will be published Jan. 23 in the journal PLOS ONE.
“When a hurricane is approaching, weather models tell us where it’s going,” said senior author Kristin Swanson, professor and vice chair of research for neurological surgery at Northwestern University Feinberg School of Medicine. “Our brain tumor model does the same thing. We know how much and where the tumor will grow. Then we can know how much the treatment deflected that growth and directly relate that to impact on patient survival.”
Swanson also is a member of the Northwestern Brain Tumor Institute and the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Maxwell Neal, lead author, is a post-doctoral researcher in bioengineering at the University of Washington.
The method will advance brain tumor treatment, Swanson said, by helping distinguish effective treatments from ineffective ones and enabling clinicians to optimize treatment plans on a patient-by-patient basis.
Muddy Zone Right After Treatment
“There is this muddy zone right after the first round of treatments when it’s hard for the clinician to know whether to change therapy because she doesn’t have the metrics that correlate to outcome,” Swanson said. “The doctor can’t yet gauge how much it helped.”
If the doctor determines the treatment isn’t effective, she can try a different type of treatment or help the patient enroll in a clinical trial with a new drug being tested. The information also is helpful to the patient.
“The patient wants to know the therapy is doing something for them,” Swanson said. “On the flip side, if the therapy isn’t helping, then it may not be worth the side affects he is enduring.”
Not All Brain Tumors are the Same
Brain cancer patients are in great need of an approach to find optimal personalized treatments.
Brain tumors vary in their growth rate, shape and density but existing methods for measuring a treatment’s impact ignore this variation. The methods (and thus physicians) cannot distinguish between a patient with a fast-growing tumor that responds well to treatment and a patient with a slow-growing tumor that responds poorly.
By using a personalized, patient-specific approach that accounts for tumor features such as 3-dimensional shape, density and growth rate, the new Northwestern method can make this distinction.
Is it Working? How the Model Forecasts Growth and Measures Effectiveness
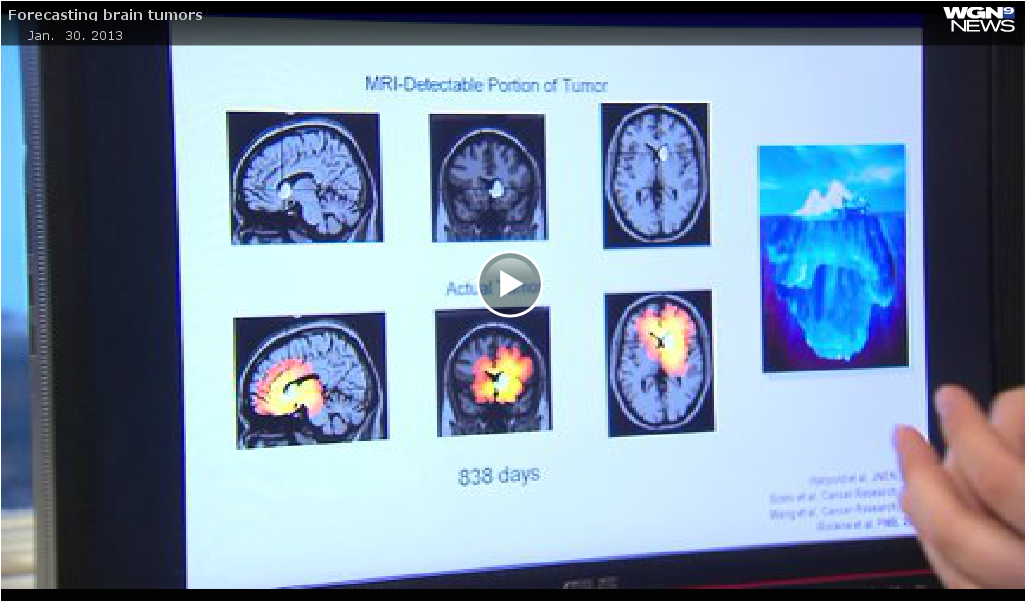
To measure a treatment’s effectiveness, the scientists performing the study created a unique computer model of each patient’s tumor and predicted how it would grow in the absence of treatment, explained Neal.
The prediction model was based on the MRI scans that the patient received on the day of diagnosis and on the day of surgery. The difference between these two scans enabled researchers to estimate how fast the tumor was growing along with the density of tumor cells throughout the brain.
Researchers then scored the effectiveness of the patient’s treatment by comparing the size of the patient’s tumor after treatment to the model-predicted size if untreated.
“The study demonstrated that higher-scoring patients survived significantly longer than lower-scoring patients and their tumors took significantly longer to recur,” Neal said. “The score can guide clinicians in determining the effectiveness of the therapy.”
Northwestern researchers hope to make the computer model an iPad app or offer it on a website where a clinician can simply enter a patients’ MRI data to calculate the response score.
The research was supported by the National Cancer Institute of the National Institutes of Health, grants R01 CA164371, R01 NS 060752, U54 CA143970. In addition, the research was funded by the McDonnell Foundation, the Brain Tumor Funders Collaborative, the University of Washington Academic Pathology Fund, and the James D. Murray Endowed Chair.
NORTHWESTERN NEWS: https://www.eurekalert.org/pub_releases/2013-01/nu-fbt011813.php