Come join us for snacks and drinks at a reception to hear about the Comprehensive NeuroOncology Program at Mayo Clinic in Phoenix including:
• Scientific Updates from Mayo Faculty – including a poster session
• Tour of the Cancer Center and Proton Beam
• Hosted in the Precision Neurotherapeutics Lab.
Bus pick up at the SNO conference venue JW Marriott Desert Ridge at 5:15pm.
News
Dr. Swanson's Twitter TimelineLab Open House During The Annual Society for NeuroOncology Meeting
Our latest paper exploring the role of differential blood-brain barrier permeability and therapeutic sensitivity in glioblastoma treatment response is up on bioRxiv
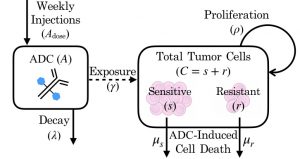 When a drug fails, often a mismatch between drug and target is blamed, and in brain tumors, there is also a possibility of insufficient amounts of drug being delivered to all of the brain tumor. In dense tumor regions, there is a break down of the blood-brain barrier due to tumor angiogenesis, which may allow drugs to enter the tumor. However, this does not occur in the outer regions of tumor where smaller numbers of tumor cells invade and spread outward, potentially leaving a large portion of tumor undertreated. To explore these dynamics, MNO lab postdoc Susan Massey and recent ASU graduate Javi Urcuyo developed an ordinary differential equation model based on experimental data from our Mayo Clinic colleague Jann Sarkaria’s Translational Neuro-Oncology lab. Our model shows how overall exposure to treatment may be more influential than therapeutic resistance in determining the therapeutic outcome.
When a drug fails, often a mismatch between drug and target is blamed, and in brain tumors, there is also a possibility of insufficient amounts of drug being delivered to all of the brain tumor. In dense tumor regions, there is a break down of the blood-brain barrier due to tumor angiogenesis, which may allow drugs to enter the tumor. However, this does not occur in the outer regions of tumor where smaller numbers of tumor cells invade and spread outward, potentially leaving a large portion of tumor undertreated. To explore these dynamics, MNO lab postdoc Susan Massey and recent ASU graduate Javi Urcuyo developed an ordinary differential equation model based on experimental data from our Mayo Clinic colleague Jann Sarkaria’s Translational Neuro-Oncology lab. Our model shows how overall exposure to treatment may be more influential than therapeutic resistance in determining the therapeutic outcome.
S. C. Massey, J. C. Urcuyo, B. M. Marin, J. N. Sarkaria, K. R. Swanson. Quantifying glioblastoma drug response dynamics incorporating resistance and blood brain barrier penetrance from experimental data. Bulletin of Mathematical Biology, in review. BioRxiv doi: 10.1101/822585
Dr Pamela R. Jackson wins People’s Choice Poster Award at the NCI PSON Meeting in Minneapolis
 Pamela R. Jackson received the People’s Choice award for her poster at the NCI Physical Sciences-Oncology Network Annual Investigators Meeting entitled T2-weighted Imaging May Be Indicative of Drug Distribution in Glioblastoma Patients. This work is a result of the NIH-funded PSOC alongside MIT.
Pamela R. Jackson received the People’s Choice award for her poster at the NCI Physical Sciences-Oncology Network Annual Investigators Meeting entitled T2-weighted Imaging May Be Indicative of Drug Distribution in Glioblastoma Patients. This work is a result of the NIH-funded PSOC alongside MIT.
2018 Newsletter
This year we have put together a year end summary/newsletter to keep you apprised of our ongoing efforts and new endeavors

Julia Lorence receives First Place “Best Research Poster”

Julia Lorence receives First Place for her thesis abstract on “Female Glioblastoma Patients Receiving Standard of Care Survive Longer”at 2018 ASU West Pre‐Health Symposium Research Poster Session
MNO Lab Presents 7 Posters at Mayo Clinic Young Investigators Research Symposium
 In March of 2018, seven MNO lab members presented posters at the Mayo Clinic Young Investigators Research Symposium in Rochester, MN. The group included Aditya Khurana (Mayo Medical School student), Cassandra Rickertsen (Image Analysis Team Lead), Gustavo De Leon (post-baccalaureate), Susan Massey (post-doctoral researcher), Sara Yee (ASU undergraduate), Julia Lorence (ASU undergraduate), and Paula Whitmire (post-baccalaureate). Gustavo De Leon was selected to give a poster teaser presentation of his research on identifying early indicators of immunotherapeutic response in GBM patients. The event served as an excellent opportunity for our young investigators to present their research and network with investigators from the other Mayo Clinic campuses.
In March of 2018, seven MNO lab members presented posters at the Mayo Clinic Young Investigators Research Symposium in Rochester, MN. The group included Aditya Khurana (Mayo Medical School student), Cassandra Rickertsen (Image Analysis Team Lead), Gustavo De Leon (post-baccalaureate), Susan Massey (post-doctoral researcher), Sara Yee (ASU undergraduate), Julia Lorence (ASU undergraduate), and Paula Whitmire (post-baccalaureate). Gustavo De Leon was selected to give a poster teaser presentation of his research on identifying early indicators of immunotherapeutic response in GBM patients. The event served as an excellent opportunity for our young investigators to present their research and network with investigators from the other Mayo Clinic campuses.
Simulated birth and 3D brain mass part of AHCJ field trip tour
https:://healthjournalism.org/blog/2018/04/simulated-birth-and-3d-brain-mass-part-of-ahcj-field-trip-tour/
April Fleming and Kyle Singleton present at 2017 Biomedical Engineering Society Annual Meeting in October 2017
 April Fleming, an ASU summer undergraduate intern with the PNT program, presented a poster on her work “Mathematical model of brain tumor growth facilitates tumor cell quantification from bioluminescence imaging”. Kyle W. Singleton, PhD, presented a poster “Comparison Of Brain Tumor Segmentation Methods For Computing Volumetric and Radial Measurements”.
April Fleming, an ASU summer undergraduate intern with the PNT program, presented a poster on her work “Mathematical model of brain tumor growth facilitates tumor cell quantification from bioluminescence imaging”. Kyle W. Singleton, PhD, presented a poster “Comparison Of Brain Tumor Segmentation Methods For Computing Volumetric and Radial Measurements”.
NIH Awards MNO and Collaborators $3.4 Million Glioblastoma Grant
NIH Awards Mayo Clinic Researchers $3.4 Million Glioblastoma Grant
The National Institutes of Health (NIH) awarded Mayo Clinic researchers in Arizona a $3.4 million grant to study how mathematical modeling can be used to help treat patients with glioblastoma – the most common type of malignant brain cancer. Glioblastomas are made up of many different cell types and tumor cell subtypes. These cells can invade far into the brain and well beyond where the tumor can be seen on clinical imaging, such as MRIs. Surgical removal of these invasive tumor cells is risky. Yet, little is known about these residual tumor cells and how best to treat them using other treatments such as radiation or chemotherapy.
Kristin Swanson, Ph.D., vice chair of research at Mayo Clinic’s Department of Neurosurgery is utilizing mathematical modeling to extract new information from MRI scans to unlock clues for how to best treat these residual tumor cells. Achieving this project requires a team with complementary skills. Swanson teamed with neuroradiologist, Leland Hu, M.D., molecular biologist, Nhan Tran, Ph.D. and imaging informaticist, Ross Mitchell, Ph.D. Using MRI data; their team produces maps of the different tumor subtypes found within a patient’s brain. “MRI-based mathematical models can be used to predict genomic content of these invasive tumor regions. These models provide a non-invasive way to identify the different tumor subpopulations in this invasive region for each patient. If we know the genetic content of the different parts of each patient’s tumor, we can match treatments that target each of the different genetic abnormalities,” says Dr. Swanson, whose team also leverages genomics, computer vision and artificial intelligence as part of their approach. Dr. Swanson says these new mathematical models can be applied to each patient’s MRIs over time, providing crucial data on how tumor cells grow or respond to treatment in each patient.
“This knowledge allows us to better advance individualized medicine”
“This knowledge allows us to better advance individualized medicine,” says Dr. Swanson. “We can better match each patient with the combination of treatments that will best target the different populations of tumor cells within each tumor. This information may also reveal new treatment targets, open up additional treatment pathways and improve a physician’s ability to monitor the effects of treatment.”


